Mixed ionic covalent compound naming is an essential aspect of chemistry, providing a systematic approach to identify and classify these unique compounds. Unlike purely ionic or covalent compounds, mixed ionic covalent compounds exhibit a fascinating blend of properties, making their naming a subject of both intrigue and importance.
This comprehensive guide will delve into the intricacies of mixed ionic covalent compound naming, equipping you with a thorough understanding of the concepts, conventions, and exceptions that govern this field.
Mixed Ionic Covalent Compound Naming Basics

Mixed ionic covalent compounds, also known as amphoteric compounds, exhibit characteristics of both ionic and covalent bonds. They are formed when a metal and a non-metal react, resulting in a compound with a partially ionic and partially covalent bond.
Characteristics
- Possess both ionic and covalent bonds within the same compound.
- Exhibit properties intermediate between ionic and covalent compounds.
- Typically have higher melting and boiling points than covalent compounds but lower than ionic compounds.
- Tend to be soluble in both polar and nonpolar solvents.
Naming Conventions
Mixed ionic covalent compounds follow specific naming conventions to accurately represent their chemical composition. These compounds consist of both ionic and covalent bonds, necessitating a systematic approach to naming.
Step-by-Step Guide
To name a mixed ionic covalent compound, follow these steps:
- Identify the cation (positive ion) and anion (negative ion) in the compound.
- Name the cation first, followed by the anion. The cation name remains unchanged, while the anion name is modified to end in “-ide.”
- For complex anions, enclose the anion name in parentheses and add the suffix “-ate” or “-ite” to indicate its charge.
- Use Roman numerals to specify the oxidation state of the metal ion if it can exhibit multiple oxidation states.
Examples
Here are examples of different types of mixed ionic covalent compounds and their correct names:
- NaCl: Sodium chloride (ionic compound)
- CaO: Calcium oxide (ionic compound)
- Fe 2O 3: Iron(III) oxide (mixed ionic covalent compound)
- CuSO 4: Copper(II) sulfate (mixed ionic covalent compound)
- K 2Cr 2O 7: Potassium dichromate (mixed ionic covalent compound)
Oxidation State Determination: Mixed Ionic Covalent Compound Naming
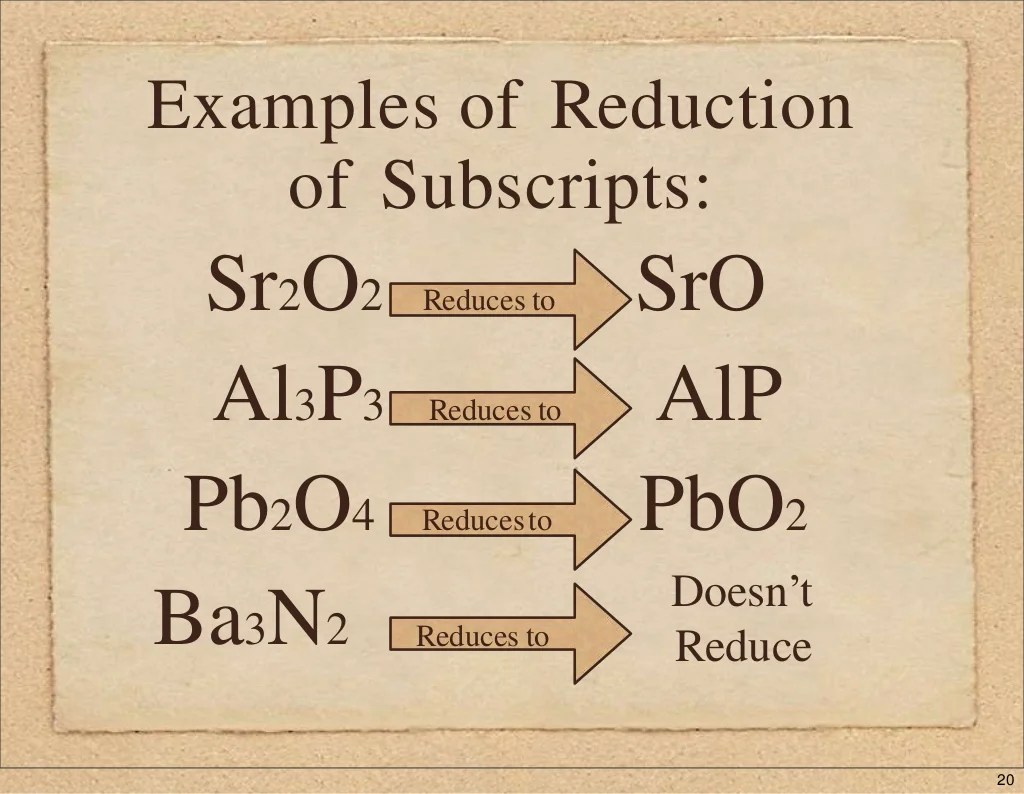
In mixed ionic covalent compounds, the oxidation state of each element plays a crucial role in determining the compound’s name and formula.
The oxidation state of an element represents its hypothetical charge if all its bonds were completely ionic. It provides insights into the element’s bonding behavior and helps establish the overall charge balance within the compound.
Methods for Determining Oxidation State
Several methods can be employed to determine the oxidation state of elements in mixed ionic covalent compounds:
- Binary Compounds:In binary compounds containing a metal and a non-metal, the metal typically has a positive oxidation state equal to its group number, while the non-metal has a negative oxidation state equal to its group number minus 8.
- Oxidation Number Rules:Specific rules govern the oxidation states of certain elements in various compounds:
- Group 1 metals (alkali metals) have an oxidation state of +1.
- Group 2 metals (alkaline earth metals) have an oxidation state of +2.
- Hydrogen has an oxidation state of +1 when bonded to non-metals and -1 when bonded to metals.
- Oxygen has an oxidation state of -2 in most compounds.
- Fluorine has an oxidation state of -1 in all compounds.
- Balancing Redox Reactions:In redox reactions, the oxidation states of elements change. By balancing the reaction and ensuring that the total oxidation state of the reactants equals the total oxidation state of the products, the oxidation states of individual elements can be determined.
Determining the oxidation state of elements is essential for accurately naming mixed ionic covalent compounds and understanding their chemical properties.
Common Examples

Mixed ionic covalent compounds are prevalent in various industries and applications. These compounds exhibit unique properties that make them indispensable for specific purposes.
Properties and Applications
Mixed ionic covalent compounds typically possess a combination of properties, including:
- High melting and boiling points due to strong intermolecular forces
- Solubility in both polar and nonpolar solvents
- Reactivity with both acids and bases
These properties make mixed ionic covalent compounds useful in a wide range of applications, such as:
- Catalysts in chemical reactions
- Electrolytes in batteries
- Pigments in paints and dyes
- Pharmaceuticals
Table of Common Examples
The following table lists some common mixed ionic covalent compounds, along with their chemical formulas, names, and brief descriptions:
| Chemical Formula | Name | Description |
|---|---|---|
| NaCl | Sodium chloride | Common table salt, used as a food additive and preservative |
| KCl | Potassium chloride | Used as a fertilizer and in medicine to treat electrolyte imbalances |
| CaCO3 | Calcium carbonate | Found in limestone, chalk, and seashells; used as a building material and in antacids |
| MgO | Magnesium oxide | Used as a refractory material in furnaces and as an antacid |
| Fe2O3 | Iron(III) oxide | Commonly known as rust; used as a pigment in paints and as a polishing agent |
Exceptions and Special Cases
Exceptions to the general naming rules for mixed ionic covalent compounds arise due to specific chemical properties or historical conventions. These exceptions are essential to understand for accurate and consistent compound naming.
Compounds with Variable Oxidation States
Some metal ions can exhibit variable oxidation states, leading to exceptions in naming. For example, iron can form compounds with oxidation states of +2 and +
3. The naming convention reflects this variability
- Iron(II) chloride (FeCl 2) for the +2 oxidation state
- Iron(III) chloride (FeCl 3) for the +3 oxidation state
Compounds with Complex Anions
Certain anions, known as complex anions, have unique names that deviate from the general naming rules. Examples include:
- Nitrate (NO 3–)
- Sulfate (SO 42-)
- Phosphate (PO 43-)
These anions are named based on their molecular structure and historical usage.
Compounds with Organic Ligands
When mixed ionic covalent compounds contain organic ligands (molecules containing carbon), the naming conventions follow specific rules. The organic ligand is named as a prefix, followed by the metal ion name and the anion name. For example:
- Potassium acetate (CH 3COOK)
- Sodium oxalate (Na 2C 2O 4)
Historical Names, Mixed ionic covalent compound naming
Some mixed ionic covalent compounds have retained historical names that differ from the systematic naming rules. Examples include:
- Water (H 2O)
- Ammonia (NH 3)
- Methane (CH 4)
These historical names are widely recognized and accepted in scientific communication.
Frequently Asked Questions
What are the key characteristics of mixed ionic covalent compounds?
Mixed ionic covalent compounds possess characteristics of both ionic and covalent compounds. They typically exhibit partial ionic character due to the presence of polar bonds, but also display covalent character due to the sharing of electrons between atoms.
How is the oxidation state determined in mixed ionic covalent compounds?
The oxidation state of elements in mixed ionic covalent compounds can be determined using various methods, such as the oxidation number method or the electronegativity equalization method.
What are some common exceptions to the general naming rules for mixed ionic covalent compounds?
Exceptions to the general naming rules arise in certain cases, such as when the compound contains a metal that exhibits multiple oxidation states or when the compound has a complex or unusual structure.